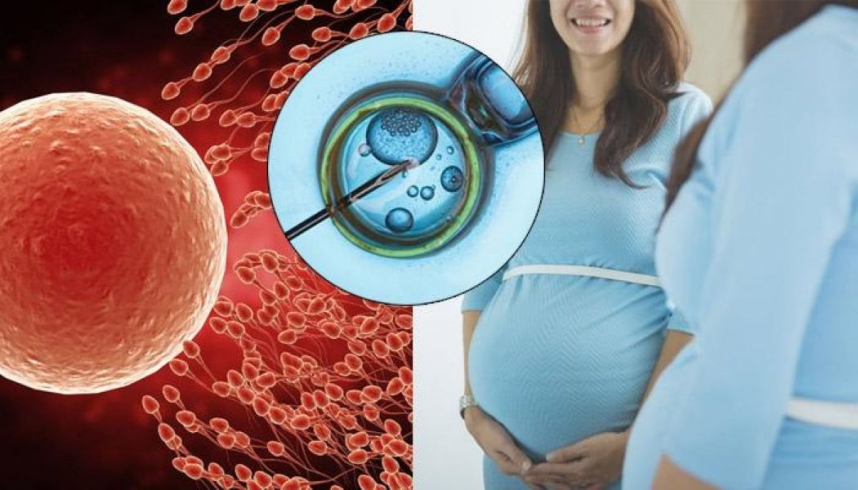
An experimental oral drug has shown promise for improving successful pregnancy rates in vitro fertilization in a mid-stage trial, researchers reported on Sunday at the European Society of Human Reproduction and Embryology Annual Meeting (ESHRE) in Amsterdam.
The researchers said the first-of-its-kind non-hormonal medication increased embryo implantation, pregnancy, and live birth rates.
The drug, OXO-001 from Barcelona-based biotech Oxolife SL, acts directly on the endometrium, the lining inside the uterus, preparing it to receive the embryo.
The 96 women in the trial, who each had a single embryo transferred into the uterus after IVF, received either the daily pill every morning starting one menstrual cycle before the embryo transfer cycle and continuing until five weeks after the transfer, or they got a placebo.
Clinical pregnancy rates, with a fetal heartbeat evident at 5 weeks after embryo transfer, were 50% with OXO-001 vs 35.7% with placebo.
At 10 weeks after embryo transfer, ongoing pregnancy rates were 46.3% with OXO-001 vs 35.7% in the placebo group.
“Most importantly, there was an absolute increase... in live birth rates” with OXO, at 42.6%, versus 35.7% with placebo, the researchers said.
At six months of age, the babies born to women in the study were all developing well, they said.
Until now, advances in improvements in live birth rates in medically assisted reproduction have been “incremental at best,” Dr Karen Sermon, Chair of ESHRE, who was not involved in the study, said in a statement.
“A jump of nearly 7% is excellent news for our patients, and hopefully this can be confirmed in larger patient groups.”